Table of Contents
Advertisement
Quick Links
C41V1 Probe
INSTRUCTION MANUAL
Notes for operators and responsible maintenance personnel
★ Please read through this Instruction Manual carefully prior to use.
★ Keep this Instruction Manual together with the system with care to
make it available anytime.
Tokyo , Japan
Q1E-EP1432-7
© Hitachi, Ltd. 2013,2017. All rights reserved.
0123
Advertisement
Table of Contents

Summary of Contents for Hitachi C41V1
- Page 1 Notes for operators and responsible maintenance personnel ★ Please read through this Instruction Manual carefully prior to use. ★ Keep this Instruction Manual together with the system with care to make it available anytime. Tokyo , Japan Q1E-EP1432-7 © Hitachi, Ltd. 2013,2017. All rights reserved. 0123...
- Page 2 Hitachi, Ltd. 2-16-1, Higashi-Ueno, Taito-ku, Tokyo,110-0015, Japan +81-3-6284-3668 http://www.hitachi.com/businesses/healthcare/ index.html European Representative: Hitachi Medical Systems GmbH Otto-von-Guericke-Ring 3 D-65205 Wiesbaden, Germany EU Importer: Hitachi Medical Systems Europe Holding AG Address: Sumpfstrasse 13 CH-6300 Zug, Switzerland Local Distributor: ( 1 ) Q1E-EP1432...
- Page 3 About this manual This instruction manual contains safety precautions, the inspection, the operation procedure and the reprocessing procedure of C41V1 Probe. Please read this manual thoroughly to ensure the safety operation. If you have any questions concerning the operation of the probe, please contact a service support.
- Page 4 Graphical Symbols for Use in Labeling of Hitachi Ultrasound Probes Some graphical symbols that are used in labeling of Hitachi Ultrasound Probes are compliant with EN980:2008 standard. Refer to the following table about the meanings of them. Explanation of Symbol...
- Page 5 Definition of symbol The following symbol is also used for HITACHI Ultrasound Probes. Location Symbol Definition This instrument complies with Directive 93/42/EEC relating to Probe connector Medical Device and Directive 2011/65/EU relating to RoHS IPX7 mark IPX7 Probe connector See section 1.6.
-
Page 6: Table Of Contents
3.1 Connection and Settings .......... 6 3.2 How to attach the Sterile Puncture Adapter (EZU-PA7V) .... 8 3.3 Display of Needle Guide Line ........9 4. Option of C41V1 Probe ........11 4.1 Magnetic sensor ..........11 5. Cleaning, Disinfection and Sterilization ....13 5.1 Point of use (Pre-cleaning) ........ -
Page 7: Introduction
1. Introduction 1.1 Features C41V1 Probe is a Convex Array Probe. The acoustic output of this probe when connected to ultrasound diagnostic scanner was measured according to the IEC60601-2-37 standard. The table of measured acoustic output data is contained in the operational manual of each ultrasound diagnostic scanner. -
Page 8: Intended Use
1.3 Intended Use C41V1 Probe is designed for observation and diagnosis of the following regions mainly by connecting with the HITACHI ultrasound diagnostic scanner. General OB/GYN organs Biopsy (with a Sterile Puncture Adapter) Transvaginal/Transrectal WARNING Never use the probe for following regions. -
Page 9: Accessories (Option)
1.5 Accessories (Option) 1.5.1 Sterile Puncture Adapter EZU-PA7V (Disposable) EZU-PA7V is the attachment for ultrasound guided transvaginal or transrectal biopsy and aspiration of organs, cyst and tumor. The size of needles available is 16 to 19G. Biopsy application requires special care. 24 pieces of Sterile Puncture Adapter EZU-PA7V are packaged in one set as follows: Component Model... -
Page 10: Construction
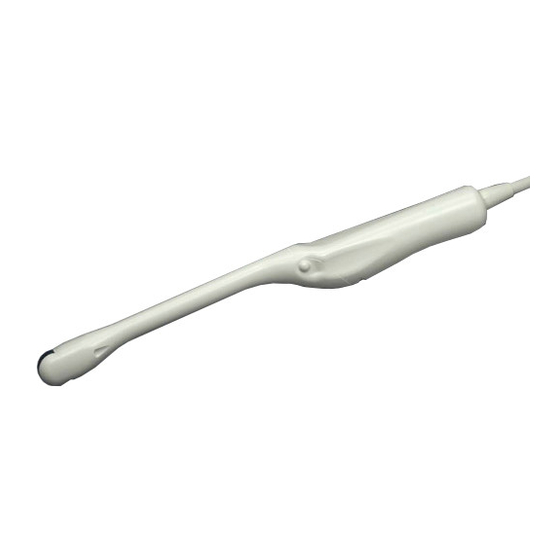
1.6 Construction The external view of C41V1 Probe is shown in Fig.1. Immersible part (IPX7) Cable Applied part Connector Magnetic sensor attachment (Option) Un-immersible part Immersible part: This part can be immersed in disinfectant solution and also can be cleaned by water. -
Page 11: Inspection Before Use
2. Inspection before Use Prior to use, the probe and accessories must be carefully inspected that they are appropriate for use. If you find any damage, do not use them and contact a service support immediately. 2.1 Inspection for Appropriate Connection 2.1.1 Confirm that the ultrasound diagnostic scanner is correctly operating. -
Page 12: Operation Procedure
The Magnetic sensor attachment is needed for Real-time Virtual Sonography(RVS). Regarding the option for RVS, please refer to “4.Option of C41V1 probe”. 2) It is recommended to use a disposable probe cover for Orientation mark preventing a patient from... - Page 13 5) Putting a probe cover onto the probe is recommended. If using probe cover, draw the probe cover until the end of the probe shaft. If air bubbles remain in jelly, remove air bubbles by pushing jelly with finger. (See Fig.4) Probe cover Air bubble Sterilized acoustic jelly...
-
Page 14: How To Attach The Sterile Puncture Adapter (Ezu-Pa7V)
8) After using the probe, clean, disinfect and if necessary sterilize the probe immediately. If RVS is carried out, clean and disinfect and if necessary sterilize the Magnetic sensor attachment. 9) Store the probe and the Magnetic sensor attachment under the conditions written in “6. -
Page 15: Display Of Needle Guide Line
3.3 Display of Needle Guide Line The needle guideline in dot marks can be displayed on the monitor for biopsy. Regarding the needle guideline, refer to the operation manual of the ultrasound diagnostic scanner. NOTE: The needle guide line is intended to provide a visual guide to the direction of the puncture needle pathway. - Page 16 WARNING 1) Special attention should be paid for a probe cover made of latex. Latex may cause allergic reactions such as itching, rubor, urticaria, swelling, fever, anhelation, wheezing, depression of blood pressure, and shock. For the patients suspected of latex allergy, do not use the latex-containing medical devices.
-
Page 17: Option Of C41V1 Probe
4. Option of C41V1 Probe 4.1 Magnetic sensor 4.1.1 How to attach the Magnetic sensor The procedure of attaching the magnetic sensor is as follow. Confirm that the Magnetic sensor attachment is sterilized or disinfected. Insert Magnetic sensor into the Magnetic sensor attachment in the correct direction as shown in Fig.7. - Page 18 CAUTION Do not put your fingers between the Magnetic sensor attachment and the probe when attaching the Magnetic sensor attachment to the probe. 4.1.2 How to release the Magnetic sensor The procedure of releasing the magnetic sensor is as follow. Detach the Magnetic sensor attachment from the probe as shown in Fig.9.
-
Page 19: Cleaning, Disinfection And Sterilization
5. Cleaning, Disinfection and Sterilization The probe and accessory must be reprocessed after each use. Refer to the reprocessing instruction in this chapter. ‐ The probe is delivered unsterile. Prior to the first use, reprocess the probe. ‐ Temperature should not exceed 60°C during WARNINGS reprocessing. - Page 20 Application part contacts Disinfection semicritical mucosa (intracavitary (Disinfectant with application) virucidal effect) Cleaning Disinfection Application part contacts (Disinfectant with intracorporeal tissue critical virucidal effect - directly minimum) (operative application) Sterilization According intended use, C41V1 probe classified semicritical. - 14 - Q1E-EP1432...
- Page 21 The flowchart of the reprocessing process of this probe is as follows. Point of use (Pre-cleaning) Containment and transportation Manual cleaning and disinfection Manual Cleaning Rinsing after manual cleaning Manual Disinfection Rinsing after manual disinfection Drying Packing Sterilization - 15 - Q1E-EP1432...
-
Page 22: Point Of Use (Pre-Cleaning)
(Pre-cleaning) Pre-cleaning should be done immediately after each use. The procedure is as follows: A) C41V1 probe 1) Remove the protective cover. 2) Clean the probe of all patient’s blood or fluid with running tap water until the surface of the probe looks visually clean. -
Page 23: Containment And Transportation
Containment and Containment and transportation transportation Putting the contaminated equipment into exclusive shock and damage proof container for transportation is recommended. It is recommended that instruments are reprocessed as soon as possible and not later than 4 hours after usage. Manual Cleaning and disinfection Prepare following... - Page 24 Prepare the detergent solution in a tank with cold water (please follow the instructions of the detergent manufacturer regarding application, dilution and contact time). A) C41V1 probe The temperature of the detergent solution should be between 15-30 °C, concentration is 1.6%. Please note the minimum contact time of the detergent in the manufacturer’s instruction.
- Page 25 B) Magnetic sensor attachment The temperature of the detergent solution should be between 15-30 °C, concentration is 1.6%. Please note the minimum contact time of the detergent in the manufacturer’s instruction. If a differing detergent is used, please also note the approved material compatibility for the medical device.
- Page 26 Manual disinfection: A) C41V1 probe 1) Prepare the disinfectant solution in a tank with cold water (please follow the instructions of the disinfectant manufacturer regarding application, concentration, microbiological efficiency, service life and contact time). 2) Confirm the concentration of the disinfectant before immersing the probe.
-
Page 27: Drying
4) Visually check the outer surface of the Magnetic sensor attachment for leavings of the disinfectant. If necessary, repeat the rinsing. Water, Detergent or Disinfectant Fig.11 Immersion of the Probe and the Magnetic sensor attachment Drying Drying 1) Wipe the equipment with a single-use, fluff-free wipe or towel to remove moisture from the surface of the equipment. -
Page 28: Packaging
Packaging Packaging Pack the equipment in a sterile barrier such as Polypropylene fleece or transparent package made from Polyethylene film and Tyvek®, and then place it into a tray. The tray should be also covered with a sterile barrier. Additionally the equipment can be placed on plastic mesh wires supplied for plasma sterilization and then packed as mentioned above. -
Page 29: Sterilization
Sterilization Sterilization The probe and accessory can be sterilized using either ethylene oxide gas (EtO) sterilization or plasma sterilization (see table below). Sterilization Method Probe and Magnetic sensor attachment Plasma Sterilization Applicable ETO Sterilization Applicable Follow the manufacturer's instructions of the sterilizer regarding usage, temperature and sterilization-time. - Page 30 The packaging procedure is as follows. 1) Put the probe into TYVEK pouch. TYVEK Pouch Fig.12 Packaging in the pouch 2) Seal the TYVEK Pouch using a heat sealer. Ensure that the seal is complete. Sealed TYVEK Pouch Probe Fig.13 Sealing - 24 - Q1E-EP1432...
-
Page 31: Storage
3) Put the sealed pouch into a tray or plastic mesh wire for sterilization. Tray for Probe in sterilization the Pouch Fig.14 Packaging in a tray Storage Store the equipment in a cool, dustproof and dark, dry space to avoid high temperature, humidity... -
Page 32: Maintenance And Safety Inspection
6. Maintenance and Safety Inspection 6.1 Daily Inspection 6.1.1 Visually inspect the surface of the probe head, housing, cable and connector for any crack, scratch or denaturalization. If you find any damage, do not use the probe and contact a service support immediately. 6.1.2 Visually inspect the surface of the Magnetic sensor attachment for any crack, deformation or denaturalization. -
Page 33: Safety Precautions
The latex probe cover may cause allergic reactions such as itching, rubor, urticaria, swelling, fever, anhelation, wheezing, depression of blood pressure, and shock. The ultrasound jelly which is an accessory of Hitachi ultrasound scanner is not sterile jelly, so never use it for C41V1. - Page 34 CAUTION When the probe is used for surgical or minimal-invasive procedures, keep electrocautery away from the probe. When defibrillator is used, take the probe out of the body. Keep the acoustic power low and minimize the ultrasound exposure time for the examination of an early pregnancy. Do not expose the connector to water or other liquids.
-
Page 35: Specifications
8. Specifications 8.1 Probe Type : C41V1 Probe Acoustic working frequency : 6.5MHz Technology : Convex Array Probe Dimensions : See Fig.15 Weight : Approx.0.80kg (Including cable and connector) Probe materials : Bio-compatible allergy free components Acoustic output : According to IEC 60601-2-37 (See Main Unit manual.) -
Page 36: Sterile Puncture Adapter Ezu-Pa7V
8.2 Sterile Puncture Adapter EZU-PA7V Type : EZU-PA7V External view : See Fig.16 Acceptable needle gauge : 16G to 19G Materials : Bio-compatible allergy free components Classification : MDD classification IIa Package : 24 Sterile Puncture Adapters for single use Sterilization method : Sterilized with gamma irradiation Operating conditions:... -
Page 37: Suppliers List
8.3 Suppliers List The products listed below are seriously tested and approved for use with C41V1 Probe. Product name manufacturer purpose Cidezyme® Johnson & Johnson Enzymatic detergent STERANIOS 2% ANIOS Disinfectant ANIOXYDE1000 ANIOS Disinfectant CIDEX Johnson & Johnson Disinfectant CIDEX® plus™ 28 Johnson & Johnson Disinfectant CIDEX®... - Page 38 Fig.15 External view of C41V1 Probe - 32 - Q1E-EP1432...
- Page 39 Fig.16 External view of C41V1 Probe with Sterile Puncture Adapter (EZU-PA7V) - 33 - Q1E-EP1432...












